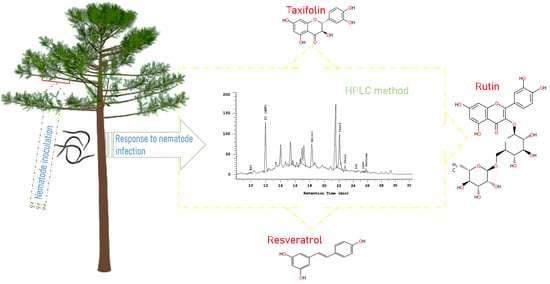
Phenolic Compounds Regulating the Susceptibility of Adult Pine Species to Bursaphelenchus xylophilus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Nematode Culture and Tree Inoculation
2.3. Tree Sampling
2.4. Extraction of Phenolic Compounds
2.5. Chemicals
2.6. Analysis of Phenolic Compounds by HPLC and LC-ESI-FT-ICR-MS
2.7. Total Phenolic Index
2.8. Statistical Analysis
3. Results
3.1. Total Phenolic Compounds
3.1.1. Non-Inoculated Trees
3.1.2. Water-Inoculated Trees
3.1.3. PWN-Inoculated Trees
3.2. Individual Phenolic Compounds
3.2.1. Non-Inoculated Trees
3.2.2. Water-Inoculated Trees
3.2.3. PWN-Inoculated Trees
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webster, J.; Mota, M. Pine Wilt Disease: Global Issues, Trade and Economic Impact. In Pine Wilt Disease: A Worldwide Threat to ForestEcosystems, 1st ed.; Mota, M., Vieira, P., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 1–3. [Google Scholar]
- Naves, P.; Bonifácio, L.; Sousa, E. The pine wood nematode and its local vectors in the Mediterranean Basin. In Insects and Diseases of Mediterranean Forest Systems; Paine, T.D., Lieutier, F., Eds.; Springer: Cham, Switzerland, 2016; pp. 329–378. [Google Scholar] [CrossRef]
- Sousa, E.; Bravo, M.A.; Pires, J.; Naves, P.; Penas, A.C.; Bonifácio, L.; Mota, M. Bursaphelenchus xylophilus (Nematoda; Aphelenchoididae) associated with Monochamus galloprovincialis (Coleoptera; Cerambycidae) in Portugal. Nematology 2001, 3, 89–91. [Google Scholar] [CrossRef]
- Naves, P.M.; Camacho, S.; De Sousa, E.M.; Quartau, J.A. Transmission of the pine wood nematode Bursaphelenchus xylophilus through feeding activity of Monochamus galloprovincialis (Col., Cerambycidae). J. Appl. Entomol. 2007, 131, 21–25. [Google Scholar] [CrossRef]
- Kuroda, K. Physiological incidences related to symptom development and wilting mechanism. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 204–222. [Google Scholar]
- Yamada, T. Biochemical responses in pine trees affected by pine wilt disease. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 223–234. [Google Scholar] [CrossRef]
- Kawaguchi, E. Relationship between the anatomical characteristics of cortical resin canals and migration of Bursaphelenchus xylophilus in stem cuttings of Pinus thunbergii seedlings. J. Jpn. For. Soc. 2006, 88, 240–244, (In Japanese with English Summary). [Google Scholar] [CrossRef]
- Ichihara, Y.; Fukuda, K.; Suzuki, K. Early symptom development and histological changes associated with migration of Bursaphelenchus xylophilus in seedling tissues of Pinus thunbergii. Plant Dis. 2000, 84, 675–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota, M.M.; Braasch, H.; Bravo, M.A.; Penas, A.C.; Burgermeister, W.; Metge, K.; Sousa, E. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- Fonseca, L.; Cardoso, J.; Lopes, A.; Pestana, M.; Abreu, F.; Nunes, N.; Mota, M.; Abrantes, I. The pinewood nematode, Bursaphelenchus xylophilus, in Madeira Island. Helminthologia 2012, 49, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Robertson, L.; Cobacho Arcos, S.; Escuer, M.; Santiago Merino, R.; Esparrago, G.; Abelleira, A.; Navas, A. Incidence of the pinewood nematode Bursaphelenchus xylophilus Steiner & Buhrer, 1934 (Nickle, 1970) in Spain. Nematology 2011, 13, 755–757. [Google Scholar] [CrossRef]
- Evans, H.F.; McNamara, D.G.; Braasch, H.; Chadoeuf, J.; Magnusson, C. Pest risk analysis (PRA) for the territories of the European Union (as PRA area) on Bursaphelenchus xylophilus and its vectors in the genus Monochamus. Bull. OEPP/EPPO Bull. 1996, 26, 199–249. [Google Scholar] [CrossRef]
- Inácio, M.; Nóbrega, F.; Vieira, P.; Bonifácio, L.; Naves, P.; Sousa, E.; Mota, M. First detection of Bursaphelenchus xylophilus associated with Pinus nigra in Portugal and in Europe. For. Pathol. 2015, 45, 235–238. [Google Scholar] [CrossRef]
- Zamora, P.; Rodríguez, V.; Renedo, F.; Sanz, A.V.; Domínguez, J.C.; Pérez-Escolar, G.; Miranda, J.; Álvarez, B.; González-Casas, A.; Mayor, E.; et al. First report of Bursaphelenchus xylophilus causing pine wilt disease on Pinus radiata in Spain. Plant Dis. 2015, 99, 1449. [Google Scholar] [CrossRef]
- Sousa, E.; Rodrigues, J.; Bonifácio, L.; Naves, P.; Rodrigues, A. Management and control of the pine wood nematode, Bursaphelenchus xylophilus, in Portugal. In Nematodes: Morphology, Functions and Management Strategies; Boeri, F., Chung, J., Eds.; Nova Science Publishers: New York, NY, USA, 2011; pp. 1–21. [Google Scholar]
- Naves, P.; Bonifácio, L.; Inácio, L.; Sousa, E. Integrated management of pine wilt disease in Troia. Rev. Ciências Agrárias 2008, 41, 4–7. [Google Scholar] [CrossRef]
- Trindade, C.S. Avaliação Da Expressão de Genes Relacionados Com a Susceptibilidade a Bursaphelenchus xylophilus, Agente Causal Da Doença Da Murchidão Dos Pinheiros (Pine Wilt Disease) Em Pinus pinaster Ait e Pinus yunannensis Franch. Master Thesis, University of Lisbon, Lisbon, Portugal, 2012; pp. 1–48. [Google Scholar]
- Son, J.A.; Matsushita, N.; Hogetsu, T. Migration of Bursaphelenchus xylophilus in cortical and xylem axial resin canals of resistant pines. For. Pathol. 2015, 45, 246–253. [Google Scholar] [CrossRef]
- Gaspar, D.; Trindade, C.; Usié, A.; Meireles, B.; Fortes, A.M.; Guimarães, J.B.; Simões, F.; Costa, R.L.; Ramos, A.M. Comparative Transcriptomic Response of Two Pinus Species to Infection with the Pine Wood Nematode Bursaphelenchus xylophilus. Forests 2020, 11, 204. [Google Scholar] [CrossRef] [Green Version]
- Nunes Da Silva, M.; Solla, A.; Sampedro, L.; Zas, R.; Vasconcelos, M.W. Susceptibility to the pinewood nematode (PWN) of four pine species involved in potential range expansion across Europe. Tree Physiol. 2015, 35, 987–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menéndez-Gutiérrez, M.; Alonso, M.; Jiménez, E.; Toval, G.; Mansilla, P.; Abelleira, A.; Abelleira-Sanmartín, A.; Díaz, R. Interspecific variation of constitutive chemical compounds in Pinus spp. xylem and susceptibility to pinewood nematode (Bursaphelenchus xylophilus). Eur. J. Plant Pathol. 2017, 150, 939–953. [Google Scholar] [CrossRef]
- Yamada, T.; Ito, S. Chemical defense responses of wilt-resistant pine species, Pinus strobus and P. taeda, against Bursaphelenchus xylophilus infection. Jpn. J. Phytopathol. 1993, 59, 666–672. [Google Scholar] [CrossRef]
- Kuroda, H.; Goto, S.; Kazumi, E.; Kuroda, K. The expressed genes of Japanese red pine (Pinus densiflora) involved in the pine wilt disease severity. BMC Proc. 2011, 5, 92. [Google Scholar] [CrossRef] [Green Version]
- López-Goldar, X.; Villari, C.; Bonello, P.; Borg-Karlson, A.K.; Grivet, D.; Zas, R.; Sampedro, L. Inducibility of plant secondary metabolites in the stem predicts genetic variation in resistance against a key insect herbivore in maritime pine. Front. Plant Sci. 2018, 9, 1651. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005, 167, 353–376. [Google Scholar] [CrossRef]
- Witzell, J.; Martín, J.A. Phenolic metabolites in the resistance of northern forest trees to pathogens—past experiences and future prospects. Can. J. For. Res. 2008, 38, 2711–2727. [Google Scholar] [CrossRef]
- Hanawa, F.; Yamada, T.; Nakashima, T. Phytoalexins from Pinus strobus bark infected with pinewood nematode, Bursaphelenchus xylophilus. Phytochemistry 2001, 57, 223–228. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Kerala, India, 2006; pp. 23–67. [Google Scholar]
- Hwang, H.; Jung, Y.H.; Yong, E.C. Enhanced accumulation of pinosylvin stilbenes and related gene expression in Pinus strobus after infection of pine wood nematode. Tree Physiol. 2021, 41, 1972–1987. [Google Scholar] [CrossRef] [PubMed]
- Futai, K. Pine wood nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modesto, I.; Sterck, L.; Arbona, V.; Gómez-Cadenas, A.; Carrasquinho, I.; Van de Peer, Y.; Miguel, C. Insights into the mechanisms implicated in Pinus pinaster resistance to pinewood nematode. Front. Plant Sci. 2021, 12, 690857. [Google Scholar] [CrossRef] [PubMed]
- Naves, P.M.; Sousa, E.; Rodrigues, J.M. Biology of Monochamus galloprovincialis (Coleoptera, Cerambycidae) in the Pine Wilt Disease Affected Zone, Southern Portugal. Silva Lusit. 2008, 16, 133–148. [Google Scholar]
- Canas, S.; Trindade, C.S.; Sun, B.; Naves, P. Phenolic compounds involved in pine wilt disease: HPLC-based method development and validation for their quantification. J. Plant Biochem. Biotechnol. 2021, 30, 343–353. [Google Scholar] [CrossRef]
- Nagle, A.M.; McPherson, B.A.; Wood, D.L.; Garbelotto, M.; Bonello, P. Relationship between field resistance to Phytophthora ramorum and constitutive phenolic chemistry of coast live oak. For. Pathol. 2011, 41, 464–469. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, C.S.; Gonçalves, E.V.; Firmino, P.N.; Calvão, T.; Fonseca, L.; Abrantes, I.; Correia, O.; Máguas, C. Differences in pine species constitutive and inducible defences determining the susceptibility to the pinewood nematode. Plant Pathol. 2016, 66, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Nose, M.; Shiraishi, S. Comparison of the gene expression profiles of resistant and non-resistant Japanese black pine inoculated with pine wood nematode using a modified Long SAGE technique. For. Pathol. 2011, 41, 143–155. [Google Scholar] [CrossRef]
- Hirao, T.; Fukatsu, E.; Watanabe, A. Characterization of resistance to pine wood nematode infection in Pinus thunbergii using suppression subtractive hybridization. BMC Plant Biol. 2012, 12, 13. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Wei, Y.; Xu, L.; Hao, Y.; Chen, X.; Zhou, Z. Transcriptomic Profiling Reveals Differentially Expressed Genes Associated with Pine Wood Nematode Resistance in Masson Pine (Pinus massoniana Lamb.). Sci. Rep. 2017, 7, 4693. [Google Scholar] [CrossRef]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, I.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity–structure relationship. J. Enzym. Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Bonello, P.; Blodgett, J.T. Pinus nigra-Sphaeropsis sapinea as a model pathosystem to investigate local and systemic effects of fungal infection of pines. Physiol. Mol. Plant Pathol. 2003, 63, 249–261. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Kandasamy, D.; Ullah, C.; Schmidt, A.; Wright, L.P.; Gershenzon, J. Flavanone-3-Hydroxylase Plays an Important Role in the Biosynthesis of Spruce Phenolic Defenses Against Bark Beetles and Their Fungal Associates. Front. Plant Sci. 2019, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Celimene, C.C.; Smith, D.R.; Young, R.A.; Stanosz, G.R. In Vitro inhibition of Sphaeropsis sapinea by natural stilbenes. Phytochemistry 2001, 56, 161–165. [Google Scholar] [CrossRef]
- Venalainen, M.; Harju, A.M.; Saranpaa, P.; Kainulainen, P.; Tiitta, M.; Velling, P. The concentration of phenolics in brown-rot decay resistant and susceptible scots pine heartwood. Wood Sci. Technol. 2004, 38, 109–118. [Google Scholar] [CrossRef]
- Suga, T.; Ohta, S.; Munesada, K.; Ide, N.; Kurokawa, M.; Shimizu, M.; Ohta, E. Endogenous pine wood nematicidal substances in pines, Pinus massoniana, P. strobus and P. palustris. Phytochemistry 1993, 33, 1395–1401. [Google Scholar] [CrossRef]
- Santhi, V.S.; Salame, L.; Muklada, H.; Azaizeh, H.; Haj-Zaroubi, M.; Awwad, S.; Landau, S.Y.; Glazer, I. Toxicity of phenolic compounds to entomopathogenic nematodes: A case study with Heterorhabditis bacteriophora exposed to lentisk (Pistacia lentiscus) extracts and their chemical components. J. Invertebr. Pathol. 2019, 160, 43–53. [Google Scholar] [CrossRef]
- Syafni, N.; Putra, D.P.; Arbain, D. 3,4-dihydroxybenzoic acid and 3,4-dihydroxybenzaldehyde from the fern Trichomanes chinense L.; isolation, antimicrobial and antioxidant properties. Indones. J. Chem. 2012, 12, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.M.; Seo, D.J.; Kim, K.Y.; Park, R.D.; Kim, D.H.; Han, Y.S.; Kim, T.H.; Jung, W.J. Nematicidal activity of 3,4-dihydroxybenzoic acid purified from Terminalia nigrovenulosa bark against Meloidogyne incognita. Microb. Pathog. 2013, 59, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Yen, G.; Duh, P.; Tsai, H. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002, 79, 307–313. [Google Scholar] [CrossRef]
- Babich, H.; Schuck, A.G.; Weisburg, J.H.; Zuckerbraun, H.L. Research strategies in the study of the pro-oxidant nature of polyphenol nutraceuticals. J. Toxicol. 2011, 2011, 467305. [Google Scholar] [CrossRef] [PubMed]
- Futai, K. Abnormal metabolites in pine wood nematode inoculated Japanese black pine. Jpn. J. Nematol. 2003, 33, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Zas, R.; Moreira, X.; Ramos, M.; Lima, M.R.M.; Nunes da Silva, M.; Solla, A.; Vasconcelos, M.W.; Sampedro, L. Intraspecific variation of anatomical and chemical defensive traits in Maritime pine (Pinus pinaster) as factors in susceptibility to the pinewood nematode (Bursaphelenchus xylophilus). Trees 2015, 29, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Daubt, M. Investigations on Pathogenicity, Invasion Biology and Population Dynamics of the Pine Wood Nematode Bursaphelenchus xylophilus (Steiner & Buhrer 1934) Nickle 1970 in European Conifers. Ph.D. Thesis, Rheinischen Friedrich-Wilhelms University, Boon, Germany, 2008; pp. 1–110. [Google Scholar]
- Mamiya, Y. Histopathological observations of Bursaphelenchus xylophilus in symptomatic tissues of pinewood. In Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems; Mota, M.M., Vieira, P.R., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 321–334. [Google Scholar] [CrossRef]







| gall | gah | ferul | fag | pca | resv | secoi | epicat | taxif | rut | quercetin h | #A | #B | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | * | * | * | * | * | * | ns | * | * | * | * | * | * | |
| T0 | P. halepensis | 0.34 ± 0.07a | 12.31 ± 1.07a | 2.02 ± 0.09a | 30.00 ± 6.17a | 4.09 ± 0.48a | 1.70 ± 0.18b | 9.18 ± 1.61 | 61.55 ± 5.67b | 24.65 ± 3.59b | 1.31 ± 0.11b | 209.22 ± 17.26b | 30.00 ± 6.17a | 68.93 ± 4.02b |
| P. pinea | 0.71 ± 0.09b | 12.39 ± 0.71a | 4.89 ± 0.34b | 18.41 ± 2.91a | 11.39 ± 0.99a | 0.51 ± 0.07a | 6.67 ± 3.07 | 12.05 ± 2.41a | 17.43 ± 1.67a,b | 0.60 ± 0.04a | 122.71 ± 9.88a | 60.28 ± 6.57b | 13.39 ± 1.91a | |
| P. pinaster | 0.80 ± 0.04b | 34.52 ± 4.46b | 5.28 ± 0.54b | 36.53 ± 5.97a | 13.42 ± 2.46a | 0.63 ± 0.08a | 6.39 ± 1.93 | 21.39 ± 2.44a | 25.51 ± 3.57b | 0.66 ± 0.03a | 210.49 ± 22.81b | 49.80 ± 3.45a | 14.26 ± 1.22a | |
| P. sylvestris | 1.90 ± 0.49b | 61.38 ± 8.62b | 6.42 ± 1.15b | 111.15 ± 16.09b | 85.25 ± 9.13b | 0.40 ± 0.08a | 1.98 ± 0.39 | 20.55 ± 1.53a | 10.29 ± 1.64a | 0.87 ± 0.11a | 97.29 ± 23.12a | 106.38 ± 5.42b | 202.06 ± 17.49c | |
| TW 24 h | Effect | * | * | * | ns | ns | * | * | * | * | * | * | * | * |
| P. halepensis | 0.65 ± 0.05a | 26.77 ± 8.34b | 2.73 ± 0.25b | 26.08 ± 3.27 | 5.86 ± 1.63 | 0.64 ± 0.11b | 9.97 ± 2.37b | 48.40 ± 7.22b | 26.32 ± 3.95b | 0.35 ± 0.01a | 255.18 ± 42.71b | 26.08 ± 3.27a | 76.32 ± 7.60b | |
| P. pinea | 0.75 ± 0.06a | 9.36 ± 1.05a | 4.75 ± 0.26c | 34.81 ± 6.23 | 3.30 ± 3.09 | 0.63 ± 0.05b | 1.53 ± 0.33a | 13.58 ± 2.88a | 17.22 ± 2.32a,b | 0.51 ± 0.05b | 192.36 ± 35.06b | 59.92 ± 3.63b | 16.55 ± 4.05a | |
| P. pinaster | 0.88 ± 0.04a | 24.71 ± 5.84b | 6.01 ± 0.17d | 26.31 ± 4.82 | 5.63 ± 1.19 | 0.74 ± 0.06b | 1.78 ± 0.20a | 14.74 ± 0.97a | 28.85 ± 5.22b | 0.64 ± 0.04b | 214.17 ± 11.29b | 57.35 ± 1.81b | 21.59 ± 1.90a | |
| P. sylvestris | 1.26 ± 0.23b | 35.53 ± 5.84b | 0.81 ± 0.41a | 23.82 ± 1.81 | 5.98 ± 0.41 | 0.23 ± 0.03a | 0.82 ± 0.19a | 29.21 ± 4.25a | 5.86 ± 2.46a | 0.61 ± 0.05b | 52.96 ± 16.63a | 62.57 ± 3.82b | 109.84 ± 19.81b | |
| TN 24 h | Effect | * | * | * | * | ns | * | * | * | * | * | * | * | * |
| P. halepensis | 0.45 ± 0.03a | 17.76 ± 3.72b | 2.78 ± 0.02a | 8.29 ± 1.83a | 2.25 ± 0.23 | 0.64 ± 0.06b | 6.82 ± 0.41c | 46.34 ± 8.88b | 14.60 ± 1.49b | 4.17 ± 0.59b | 188.62 ± 26.18b | 8.29 ± 1.83a | 69.12 ± 9.91b | |
| P. pinea | 0.74 ± 0.02b | 9.82 ± 0.93a | 4.31 ± 0.15b | 50.13 ± 3.33a,b | 2.79 ± 0.47 | 0.48 ± 0.02b | 2.72 ± 0.44a,b | 9.00 ± 2.24a | 15.30 ± 0.50b | 0.44 ± 0.03a | 174.39 ± 19.91b | 25.65 ± 3.49a | 16.12 ± 1.81a | |
| P. pinaster | 0.76 ± 0.09b | 16.45 ± 2.17a,b | 5.16 ± 0.23c | 31.08 ± 6.04a,b | 7.18 ± 2.13 | 0.54 ± 0.03b | 1.63 ± 0.08a | 16.51 ± 3.31a | 25.82 ± 2.60c | 0.51 ± 0.03a | 209.03 ± 15.19b | 55.95 ± 1.70b | 14.83 ± 1.74a | |
| P. sylvestris | 0.85 ± 0.11b | 44.13 ± 2.52c | 2.03 ± 0.49a | 89.10 ± 31.51b | 9.3 ± 3.72 | 0.21 ± 0.08a | 3.64 ± 0.86b | 26.48 ± 2.37a | 5.37 ± 0.64a | 1.12 ± 0.06a | 98.89 ± 3.75a | 75.59 ± 16.43b | 129.65 ± 21.83c | |
| TW 72 h | Effect | * | * | * | * | ns | * | * | * | * | * | * | * | * |
| P. halepensis | 0.82 ± 0.07a,b | 30.10 ± 8.75b | 3.09 ± 0.90b | 14.66 ± 1.39a | 11.99 ± 2.20 | 0.75 ± 0.07b | 5.57 ± 0.83a,b | 60.28 ± 7.77c | 24.42 ± 2.25b | 0.25 ± 0.07a | 351.61 ± 74.49b | 14.66 ± 1.39a | 95.20 ± 14.57b | |
| P. pinea | 0.52 ± 0.07a | 12.15 ± 3.06a | 3.69 ± 0.79b | 50.40 ± 5.60c | 4.51 ± 1.58 | 0.39 ± 0.07a | 1.92 ± 0.46a | 3.96 ± 0.67a | 10.29 ± 1.20a | 0.27 ± 0.06a | 121.75 ± 18.18a | 34.86 ± 3.64a | 8.68 ± 1.22a | |
| P. pinaster | 1.09 ± 0.13b | 14.66 ± 0.65a | 4.93 ± 0.18c | 27.00 ± 5.88b | 8.92 ± 2.09 | 0.70 ± 0.02b | 8.37 ± 3.33b | 12.27 ± 2.67a | 25.05 ± 1.03b | 0.55 ± 0.02b | 217.18 ± 20.18a,b | 85.25 ± 6.59b | 19.50 ± 1.71a | |
| P. sylvestris | 0.84 ± 0.19a,b | 32.59 ± 4.85b | 2.30 ± 0.17a | 31.78 ± 8.31a,b | 7.42 ± 2.19 | 0.19 ± 0.05a | 1.61 ± 0.04a | 34.72 ± 3.26b | 9.43 ± 0.76a | 0.57 ± 0.08b | 98.84 ± 3.79a | 79.04 ± 14.71b | 140.88 ± 10.46c | |
| TN 72 h | Effect | * | ns | * | ns | * | * | * | * | * | * | * | * | * |
| P. halepensis | 0.41 ± 0.02a | 24.54 ± 4.78 | 2.48 ± 2.28a | 43.67 ± 17.46 | 3.64 ± 0.64a | 1.65 ± 0.29b | 8.32 ± 0.42b | 68.04 ± 14.50b | 43.63 ± 9.25b | 4.65 ± 0.71c | 306.96 ± 94.88b | 16.10 ± 1.27a | 69.40 ± 8.42b | |
| P. pinea | 0.59 ± 0.03b | 21.04 ± 1.65 | 4.25 ± 0.54a | 69.42 ± 10.29 | 5.75 ± 2.00a | 0.49 ± 0.05a | 2.70 ± 0.0.32a | 6.49 ± 0.94a | 13.47 ± 0.66a | 0.29 ± 0.02a | 117.26 ± 3.97a | 17.17 ± 2.41a | 11.57 ± 1.10a | |
| P. pinaster | 0.91 ± 0.08b | 15.67 ± 4.97 | 6.18 ± 0.94a | 50.52 ± 8.77 | 9.21 ± 2.27a | 0.50 ± 0.04a | 1.85 ± 0.17a | 11.01 ± 1.89a | 25.96 ± 2.06a | 0.58 ± 0.05a | 210.12 ± 14.84a,b | 57.19 ± 11.32b | 13.06 ± 0.64a | |
| P. sylvestris | 2.40 ± 0.54b | 28.25 ± 7.98 | 11.36 ± 5.98b | 111.35 ± 24.94 | 47.43 ± 6.89b | 0.87 ± 0.13a | 2.60 ± 0.84a | 29.66 ± 4.91a | 12.55 ± 1.80a | 2.15 ± 0.44b | 72.02 ± 12.80a | 96.26 ± 14.34c | 177.10 ± 10.35c | |
| Interaction Sampling Time × Treatment | ||||||||||||||
| Effect | ns | ns | * | * | ns | * | ns | ns | * | ns | ns | ns | ns | |
| Interaction Species × Sampling Time | ||||||||||||||
| Effect | * | * | * | ns | * | * | ns | * | * | ns | * | * | * | |
| Interaction Species × Treatment | ||||||||||||||
| Effect | * | * | * | ns | * | * | ns | ns | ns | * | ns | * | * | |
| Interaction Species × Treatment × Sampling Time | ||||||||||||||
| Effect | * | ns | * | * | * | * | * | ns | * | ns | ns | * | ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trindade, C.S.; Canas, S.; Inácio, M.L.; Pereira-Lorenzo, S.; Sousa, E.; Naves, P. Phenolic Compounds Regulating the Susceptibility of Adult Pine Species to Bursaphelenchus xylophilus. Forests 2022, 13, 500. https://doi.org/10.3390/f13040500
Trindade CS, Canas S, Inácio ML, Pereira-Lorenzo S, Sousa E, Naves P. Phenolic Compounds Regulating the Susceptibility of Adult Pine Species to Bursaphelenchus xylophilus. Forests. 2022; 13(4):500. https://doi.org/10.3390/f13040500
Chicago/Turabian StyleTrindade, Cândida Sofia, Sara Canas, Maria L. Inácio, Santiago Pereira-Lorenzo, Edmundo Sousa, and Pedro Naves. 2022. "Phenolic Compounds Regulating the Susceptibility of Adult Pine Species to Bursaphelenchus xylophilus" Forests 13, no. 4: 500. https://doi.org/10.3390/f13040500